Search This Blog
Showing posts with label genetic. Show all posts
Showing posts with label genetic. Show all posts
Friday, July 13, 2012
Turner Syndrome
Turner syndrome is caused by a missing or incomplete X chromosome. People who have Turner syndrome develop as females. The genes affected are involved in growth and sexual development, which is why girls with the disorder are shorter than normal and have abnormal sexual characteristics.
Symptoms:
There are many different physical features associated with Turner syndrome. Not all girls have all symptoms, and in many cases the signs are hard to detect. Girls with Turner syndrome may have:
short stature (affects almost all girls with Turner, to different degrees)
failure of ovaries to develop (90-95% of girls)
webbed neck (25%) or short neck (40%)
abnormal fingernails and toenails (70%)
low hairline at neck (40%)
heart defect (30%)
kidney or urinary tract defect (30%)
hearing disorders (50-90%)
frequent ear infections in childhood (75%)
shortening of bones in the hands (35%)
lower jaw smaller than normal (60%)
drooping eyelids (ptosis), wandering eyes (strasbismus)
Girls and women with Turner syndrome have normal intelligence but often have learning problems that lead to difficulty with math and spatial relationships between objects.
Diagnosis:
If a physician suspects a girl may have Turner syndrome because she is not growing at a normal rate, and perhaps has one or more of the other signs of the syndrome, a chromosome analysis will be done. Finding the specific chromosome problem of the syndrome is the only definitive diagnosis.
Treatment:
There are two main medications given to girls with Turner syndrome. One is human growth hormone, used to increase the girl's growth rate and help her be taller. The other medication is estrogen, a female hormone, to replace the estrogen which would normally have been produced by the ovaries. Another female hormone, progesterone, is also given when the girl grows older, to help her have a normal monthly menstrual cycle.
Since a girl with Turner syndrome usually does not have ovaries, she cannot produce eggs and become pregnant when she grows up. However, some women with Turner syndrome can use in vitro fertilization to become pregnant, using donated eggs. Other women choose to adopt children in order to have a family.
source: click here
Tuesday, July 10, 2012
Danon Disease
Danon disease, a genetic disorder characterized by heart problems, mental retardation and muscle weakness. Changes in the retina of the eye, leading to visual disturbances, may be present. Males are more severely affected and show symptoms in childhood or early adolescence, while affected females usually develop the symptoms later, in adolescence or adulthood.
The disease is due to a mutation in the gene for LAMP-2 (which stands for lysosome-associated membrane protein 2.), a glycoprotein molecule (a molecule made up of carbohydrate + protein) that is normally located on the membrane surrounding the lysosome (a packet of powerful corrosive enzymes that degrade "garbage" within cells).
Danon disease is named for Dr. M.J. Danon who (together with colleagues) originally described it 1981 as "lysosomal glycogen storage disease with normal acid maltase."
Danon disease usually manifests with the clinical triad of cardiomyopathy, skeletal myopathy, and mental retardation. The skeletal myopathy and mental retardation are less common in females than in males. Regardless of sex, cardiomyopathy can present as a result of symptoms or congestive heart failure (CHF) or an arrhythmia-related event, such as syncope or sudden death. Patients are also newly identified when asymptomatic relatives of patients with established Danon disease are evaluated and are found to have the disease.
Sign and Symptoms:
* Moderate loss of central visual acuity
* Depigmentation of the peripheral retina[8]
* Decreased visual acuity with diffuse choriocapillary atrophy[11]
* Peripheral pigmentary retinopathy
* Lamellar opacities in the lens
* Nonspecific changes on electroretinography
Treatment:
Patients with Danon disease require frequent follow-up, with particular attention to the potential for atrial or ventricular arrhythmias and congestive heart failure (CHF). As is recommended in patients with hypertrophic cardiomyopathy (HCM), a ventricular septal thickness more than 30 mm is considered a risk factor for a life-threatening event, particularly in this group of patients who often have a poor prognosis for survival beyond their teenage years.
source: click here
Sunday, January 1, 2012
Juvenile Retinoschisis
Causes progressive loss of vision.
X-linked juvenile retinoschisis is a genetic disorder of the X chromosome. A gene identified as being responsible for juvenile retinoschisis, RS1, encodes an amino acid protein, retinoschisin, which is important in photoreceptor cells of the eye. Physical changes occur in the retina, the part of the eye responsible for vision. The retina splits into two layers, which impairs vision.
Since juvenile retinoschisis is an X-linked recessive disorder, it occurs primarily in boys (since they have only one X chromosome), but it can occur in females with two defective genes (one on each of their two X chromosomes). Juvenile retinoschisis occurs in individuals of all ethnic backgrounds.
Symptoms
Children with juvenile retinoschisis have gradually decreasing vision due to splitting of the retina. Physical changes in the eye may include:
* development of cysts and ruptured blood vessels between the layers of the retina
* holes in the retinal layers, which may lead to detachment of the retina from its underlying tissue (5-22% of individuals)
* leakage of blood into the jelly-like material inside the eye (vitreous hemorrhage)
* changes in the macula, the area of clearest vision in the retina.
Individuals with juvenile retinoschisis may also have difficulty focusing on an object (strabismus) and roving, involuntary eye movements (nystagmus).
Diagnosis
A thorough ophthalmological evaluation can help distinguish juvenile retinoschisis from similar retinal degenerative diseases such as retinitis pigmentosa. An imaging technique called optical coherence tomography (OCT) provides high-resolution cross-sectional images of the macula to look for abnormalities. OCT can show the changes present in juvenile retinoschisis. An electroretinogram will show dysfunction throughout the retina. Genetic testing can reveal the presence of the defective RS1 gene.
Treatment
No treatment is yet available to stop the progression of juvenile retinoschisis. Surgery can repair vitreous hemorrhage and retinal detachments. Low-vision aids, mobility training, and adaptive training skills can help individuals with vision loss. Genetic counseling can help identify family members who are carriers of the RS1 gene.
source: click here
Wednesday, September 14, 2011
Gaucher Disease

Gaucher disease is an inherited storage disorder. In Gaucher disease, a specific enzyme is deficient. This deficiency allows a fatty substance known as glucocerebroside to build up to toxic levels in the spleen, liver, lungs, bone marrow, and sometimes in the brain. Gaucher disease is genetically inherited in an autosomal recessive pattern, meaning that a child must inherit two copies of the disease gene mutation -- one from each parent -- to be born with the disease. Gaucher disease affects both males and females.
There are three types of Gaucher disease. The Type 1 form is often called the adult form because its symptoms can begin at any age. Although Type 1 occurs worldwide in people of all ethnic backgrounds, it is most prevalent in the Ashkenazi Jewish population (Jews of Eastern European ancestry). Among this group, Gaucher disease occurs at a rate of 1 in 450 births, and is the most common genetic disease affecting Jewish people.
Type 2 Gaucher disease is usually diagnosed in infancy and is the most rare form of the disease. It also occurs worldwide in people of all ethnic backgrounds, and is estimated to occur in 1 in 100,000 births.
In Type 3 Gaucher disease, symptoms appear later in childhood than in Type 2. Type 3 also occurs in people of all ethnic backgrounds. It is estimated to occur in 1 in 50,000 births.
Symptoms
The most common symptoms of Gaucher Type 1 disease are:
* enlarged liver and spleen
* fatigue due to anemia
* low blood platelets, which leads to easy bruising and nosebleeds
* bone pain
* bone deterioration and weakening (osteoporosis)
There may also be lung and kidney impairment but there is no brain involvement. In Types 2 and 3 Gaucher disease, the brain and nervous system are affected, so additional symptoms include:
* lack of coordination (ataxia)
* brain damage
* seizures
Diagnosis
A diagnosis of Gaucher disease may be suggested by the symptoms. A special blood test called an enzyme assay can measure glucocerebrosidase (GC) activity. In Gaucher disease, the GC levels will be very low, and would confirm the diagnosis.
Treatment
There is as yet no complete cure for Gaucher disease. For people with Gaucher disease Types 1 and 3, a drug called Cerezyme (imiglucerase) replaces the deficient enzyme in the disease and relieves many of its symptoms. It is important to begin this enzyme replacement therapy before there are significant organ or bone changes. The treatment can’t undo the severe brain damage that may occur in Types 2 and 3. Children with Gaucher disease Type 2 have the most severe impairment and usually do not live past 2 to 3 years of age.
Friday, July 8, 2011
Blackfan Diamond Anemia

In Blackfan Diamond (or Diamond Blackfan) anemia, the body's bone marrow produces little or no red blood cells. Blackfan Diamond anemia affects approximately 600 to 700 people worldwide. Its cause is unknown, although a genetic error in a gene called RPS19 on chromosome 19 is associated with about 25% of cases. In about 10% to 20% of cases, there is a family history of the disorder.
Symptoms:
Blackfan Diamond anemia is present at birth but can be difficult to identify. In about one-third of children born with the disorder, there are physical defects such as hand deformities or heart defects, but a clear set of signs hasn't been identified. The symptoms may also vary greatly, from very mild to severe and life-threatening.
Red blood cells carry oxygen throughout the body, so a child with Blackfan Diamond may have symptoms related to not enough blood oxygen (anemia):
* pallor (paleness)
* irregular heartbeat, due to the heart trying to keep oxygen moving throughout the body
* fatigue, irritability, and fainting.
Diagnosis:
Blackfan Diamond anemia is usually diagnosed within the first two years of life, sometimes even at birth, based on symptoms.
A complete blood cell count (CBC) for the baby would show a very low number of red blood cells as well as low hemoglobin. Another blood test would show high adenosine deaminase activity (ADA). A bone marrow sample (biopsy) would show that few new red blood cells were being created.
Treatment:
The first line of treatment for Blackfan Diamond anemia is to give the child steroid medication, usually prednisone. About 70% of children with Blackfan Diamond anemia will respond to this treatment, in which the medication stimulates the production of more red blood cells. However, this means that the child will have to take steroid medication for the rest of his or her life, which has serious side effects such as diabetes, glaucoma, bone weakening (osteopenia), and high blood pressure. Also, the medication may suddenly stop working for the person at any time.
Sunday, May 8, 2011
Angelman Syndrome

Angelman syndrome, caused by a genetic defect on chromosome 15, includes developmental delay, near absence of speech, and facial abnormalities. The most striking characteristic of someone with Angelman syndrome, though, is the appearance of being happy most of the time, with frequent smiling and prolonged episodes of laughter.
Angelman syndrome may occur in people of all ethnic backgrounds. About 70-75% of individuals born with Angelman syndrome have no family history of the disorder
Symptoms:
Individuals with Angelman syndrome share common characteristics:
* Developmental delay and functional impairment
* Disparity between understanding language and speaking; speaks few or no words; may be able to use nonverbal gestures
* Short attention span, hyperactivity, easily excitable, appears happy, frequent smiling and/or laughing
* Difficulty with movement or balance, including difficulty walking and/or tremors of limbs.
In addition, individuals with Angelman syndrome may have:
* Seizures of any type
* Delayed, disproportionate growth of head in childhood
* Hypopigmented skin and eyes
* Wide mouth, widely-spaced teeth, protruding tongue, drooling, feeding problems and frequently putting things in the mouth during infancy
* Sleep disturbance.
Diagnosis:
Since Angelman syndrome is a genetic disorder, infants are born with it. Parents begin to notice when their child is between 6-12 months of age that developmental milestones are not being met, such as sitting alone without support and standing up. Jitteriness or tremors of the limbs may be present, and once the child begins walking there may be toe-walking, lurching forward, or a jerky gait.
The child may be given the diagnosis of cerebral palsy based on these symptoms. However, the child’s behaviors of constant smiling and laughing, but not talking, point towards a diagnosis of Angelman syndrome. The diagnosis is based on the symptoms present, as there is no specific test for the syndrome.
Treatment:
Specific medical treatment may be needed for problems such as seizures, feeding problems, or sleep disturbance. Physical therapy is helpful for improving walking, and occupational therapy can help the child develop everyday living skills . The child with Angelman syndrome needs consistent behavioral management and supervision, and will require special provisions to be integrated into the classroom. Speech and communication therapy can help the child, if able, to develop nonverbal means of communication and use communication aids such as pictures to express needs. Individuals with Angelman syndrome generally have good health and can be expected to live a normal life span.
Angelman Syndrome Foundation Takes Major Step Toward Furthering Research Efforts
The Angelman Syndrome Foundation, http://www.angelman.org, announced the formation of the Angelman Treatment and Research Institute (ATRI), which will direct the organization's rapidly increasing research funding. The ATRI will also serve as a hub for more than 30 organizations, researchers and scientists worldwide to share discoveries and treatments for this neuro-genetic disorder. The announcement of the ATRI was made during the Angelman Syndrome Foundation's biennial conference in Orlando, Fla.
source: rarediseases.about.com
Friday, May 6, 2011
Inherited Ataxia Disorders
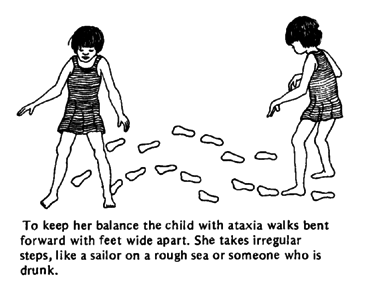
Ataxia Telangiectasia
Affects central nervous system, eyes, skin, and immunity
Ataxia telangiectasia is a genetic disorder that affects the central nervous system, the eyes, skin, and immune system. Ataxia telangiectasia affects both males and females and has been estimated to occur 1 in 40,000-50,000 individuals.
Symptoms:
1. Central Nervous System: loss of muscle control, leading to swaying of the head and trunk on standing; by age 10 children often need a wheelchair.
2.Skin: tiny red lesions, like spider veins, appear at the corners of the eyes and spread.
3. Immune System: impaired immune system leaves child open to recurrent respiratory infections.
Other symptoms may include delayed growth, difficulty speaking or swallowing, and dry coarse hair (which may be partly gray) and skin. About 20% of children with ataxia telangiectasia develop cancer such as leukemia or Hodgkin's lymphoma.
Diagnosis
Diagnosis is based on the symptoms the child has, especially the poor muscle control and the tiny red lesions on the eyes and face. The gene for ataxia telangiectasia has been identified, so genetic testing can be done to verify the diagnosis. Children are usually diagnosed sometime in early childhood (between ages 2-1/2 to 7 years old).
Treatment
There is at present no cure for ataxia telangiectasia, or way to slow down the progress of the disease. Treatment is aimed at relieving symptoms and trying to prevent respiratory infections, which are often the cause of death. Unfortunately, the outlook isn't very good; children with the disorder generally do not live beyond their teens or early 20s.
source: rarediseases.about.com
Subscribe to:
Posts (Atom)


